Tesla Battery Cell Manufacturing
The acquisition also comes at a time when its becoming obvious that Tesla is working to establish its own battery cell manufacturing capacity.
After working with Panasonic for years and now recently adding LG Chem as a supplier for battery cells, the automaker recently all but confirmed that its going to manufacture its own battery cells.
Earlier this year, Tesla acquired an ultracapacitor manufacturer called Maxwell, but it has been speculated that the acquisition is more likely related to the companys new Li-ion electrode technology.
Now with the acquisition of Hibar, Tesla also owns a company with expertise in making battery cell production equipment.
Causes Of Thermal Runaway And Energy Storage System Fires
The causes of energy storage fires and explosions are complex and can be influenced by many factors. These factors can range from errors in manufacturing, design, and installation, to human errors, but the primary cause of an energy storage system fire is caused by having a battery cell go into thermal runaway.
During thermal runaway, the heat generated within the battery cell exceeds its ability to dissipate. When this occurs, the battery cell in thermal runaway can cause adjacent battery cells to overheat, potentially driving them into thermal runaway. If this chain reaction occurs across multiple battery cells, it can spread from modules to the rack and result in a significant fire.
Thermal runaway is primarily caused by the cell undergoing external abuse conditions. These conditions include external heating, over-charging, over-discharging, high-current charging, nail penetration, crushing or the occurrence of an external short-circuit. These external abuse conditions lead to plating of the Lithium which can lead to internal short circuit of the cell.
This energized-state of the internal cell can lead to exothermic reactions within the cell such as electrode-electrolyte reactions, chemical decompositions, or other electrochemical reactions. If the heating-rate as a result of these exothermic reactions is greater than the possible dissipation rate of the cell, it may lead to thermal runaway.
Cycling And Thermal Properties Of The Electrolytes
Figure shows that the 0.93Ah Gr|NMC811 pouch cell with the concentrated LiFSI/DMC electrolyte delivered stable charge-discharge capacities for over 300 cycles at C/3. The average coulombic efficiency was 96.6% and capacity retention was 94.5% , indicated the suppressed Al dissolution and stable SEI on the graphite during cycling,,. The cell with conventional 1M LiPF6/EC:EMC electrolyte shows comparable electrochemical performances, the capacity retention after 300 cycles was 93.9%. The LiFSI/TMP concentrated electrolyte in the pouch cell was also investigated for two cycles before safety evaluation, and it delivered a coulombic efficiency of 99.5% . For the Gr|NMC532 pouch cells, the charge and discharge curves also demonstrated stable electrochemical performance with the concentrated electrolytes .
Fig. 1: Electrochemical performance and physical properties of concentrated electrolytes.
However, it was reported that the direct redox reactions between the charged cathode and anode are severe for battery chemistries of high energy density, which can cause thermal runaway even without electrolytes or ISC,. Thus, the evaluation of battery safety based on the used electrolytes only is not sufficient, and the interaction between the electrolytes and the charged electrodes should be systematically taken into consideration.
You May Like: Iphone 13 Pro Max Battery Percentage
Changing The Battery Design For Minimal Impact
According to a patent filed by Tesla, the inventors discussed a battery design that provides a predictable pathway through a portion of the cell for the efficient release of the thermal energy that occurs during thermal runaway, thereby reducing the chances of a rupture in an undesirable location.
Furthermore, the design maintains the functionality of the cell cap as the positive terminal of the cell, thereby having minimal impact on the manufacturability and maximum use in a variety of applications.
While searching for solutions to prevent thermal runaway in a lithium-ion battery, we also found some quality solutions to other problems in the LIB domain. Problems such as-
- How to overcome dendrite formation?
- How to increase the life cycle of Lithium-ion batteries?
- How to decrease viscosity and increase the dispersion efficiency of anode binders?
- How to you can make your LIB operate at high temperatures?
Seeking solutions for the above-mentioned problems? We might be able to put an end to your woes.
Contribution Of The Exothermic Reactions To Thermal Runaway

Fig. 3: Comparison of thermal runaway features of CaEly, AnEly, and CaAn partial cells with full cell.
AnEly, CaEly, and CaAn partial cells were prepared from the fully charged Gr|NMC811 batteries to investigate the contribution of different exothermic reactions during the thermal runaway process of the battery. The temperature dependence of dT/dt between the full cell and the partial cells was compared. No electrolyte in the CaAn cell, while the concentrated LiFSI/DMC electrolyte was used for all the other partial cells and the full battery.
The surge after T2 could be obviously observed in the full, AnEly, and CaAn cells. Then, the dT/dt gradually decreased until the cell temperature reached the maximum value. Although the maximum temperature of the three kinds of cells is different, the maximum dT/dt of the AnEly and CaAn cell was both brought to hundreds of orders of magnitude, which indicates that the reactions in both the AnEly and CaAn cells were mainly responsible for the exothermic reactions during thermal runaway. Also, T3 of each of the AnEly and CaAn cells was higher than that of the full cell because some of the battery components did not exist in the AnEly and CaAn partial cells.
Also Check: Solar Power Batteries For Sale
Thermal Stability Of Lifsi/dmc In Gr
A DSC-TG-MS test was used to characterize the thermal stability of the cell components. By enumerating all the thermal reactions of the individual and mixed cell components, the reactions inside the battery during the thermal runaway evolution can be screened out. As the chemical reactions in the AnEly partial cell were targeted as the trigger reaction of thermal runaway. Then, first, all the possible reactions among the lithiated anode, concentrated LiFSI/DMC electrolyte, electrolyte components, and delithiated cathode were measured .
Fig. 4: Thermal stability of cell components and their mixtures in the Gr|NMC811 battery.
a DSC traces of the lithiated anode, concentrated LiFSI/DMC electrolyte components, and their mixtures for the Gr|NMC811 battery. The inset displays the enlarged peaks of An and An+DMC. b NO2 gas evolution of LiFSI, the lithiated anode and their mixture during the DSC measurement. c SO2 gas evolution of LiFSI, the lithiated anode, and their mixture during the DSC measurement. d The weight loss of the lithiated anode, LiFSI, and their mixture. e DSC traces of the cathode, cathode mixed with concentrated LiFSI/DMC, and cathode mixed with anode.
What Causes Thermal Runaway
Several conditions can cause thermal runaway in a battery.
Thermal runaway can occur due to an internal short circuit caused by physical damage to the battery or poor battery maintenance. The same type of scenario could cause an external short circuit which could also kick off the chain reaction.
Overcharging a battery beyond its safe max voltage can permanently damage the battery and lead to thermal runaway.
Rapid charging can also lead to thermal runaway because rapid charging can lead to excessive currents.
Finally, temperatures outside of the safe region on either the low or high side degrades a batterys performance. This leads to irreversible damage to the battery and possible triggering of the reaction.
While the danger of excessive heat may be obvious, the danger of excessive cold may be confusing. The functioning of lithium-ion batteries depends on chemical reactions. Excessive cold can slow or stop those chemical reactions from occurring.
Read Also: How To Check If Car Battery Is Good
How To Prevent Thermal Runaway In A Lithium
Boeings Dreamliner 787, which Boeing advertised as 20% fuel efficient, was grounded in 2013. In the same year, Teslas Model S came under a federal safety investigation after it caught fire at least 3 times. In 2016, Samsung recalled 2.5 million Galaxy Note 7 smartphones. For all three companies, which are top players in their domain, the problem was the same thermal runaway in the Lithium-Ion battery installed in the heart of their product as a power source.
Fast forwarding to today, battery-related explosions have increased five times since 2016.
Even though lithium-ion batteries are technically safer and less likely to fail today than a decade ago, companies havent been able to completely eliminate the issue. And the use of lithium-ion batteries has increased so much more in numbers. With so many battery-operated appliances, from drones to automobiles, companies are still searching for a solution. One such company approached us with the same. Their problem statement how to stop lithium-ion batteries explosion and are there some solutions that can be adopted?
The Risk Of A Workplace Fire Sparking Thermal Runaway
Due to the risk of ambient heat causing thermal runaway in lithium-ion batteries, you should also have risk control measures in place to prevent workplace fires. Due to the elevated temperature, the fire will quickly ignite any batteries that you have onsite.
Without any control measures, the batteries will only add to the intensity of the fire and increase the risk for your business and community.
Storing batteries in a dedicated cabinet that can prevent fire from affecting the batteries can give your organisation time to action firefighting equipment or call emergency services. You should make sure that your battery cabinets are equipped with thermal protection features such as double-walled steel construction, a space of at least 40 mm between walls, and self-closing, close fitting doors.
Read Also: How To Change Mercedes Key Fob Battery
What Are The Dangers Of Ups Batteries Overheating
Left uncorrected, internal temperature will continue to increase, causing battery overheating and the outer casing to bulge, melt and/or rupture. When the casing of a VRLA battery becomes compromised, hydrogen sulfide gas will escape. If this is observed, charging power to the battery needs to be removed immediately. If undetected, thermal runaway can lead to catastrophic results, including fire, explosion, sudden system failure, costly damage to equipment, and possibly personal injury.
Thermal runaway can occur in lithium ion batteries and VLA, but this discussion focuses mainly on VRLA batteries, since they are most common in the industry. Additionally, our lithium ion battery cabinets are already equipped with . The logging and network connection/ communication capabilities allow for data-driven decision and better management of the overall system.
Heat Production In Lithium
Lithium-ion cells and electrons are responsible for producing electricity in lithium-ion batteries through their movement. With movement, comes some degree of heat which is acceptable as it can be dissipated from the cells.
However, in unusual circumstances such as lithium battery thermal runaway, heat generated is at an abnormally high rate and cannot be dissipated from the cells. The rate of heat production is much higher than that of heat dissipation.
Read Also: Does Walmart Sell Interstate Batteries
Introducing A Flame Retardant
Thermal runaway often occurs from punctures and improper charging. To counter such fire hazards, the inventors used a thermal fluid that contains a flame retardant.
A flame retardant is a compound that inhibits, suppresses, or delays the production of flames or prevents the spread of fire.
Here they have microencapsulated the flame retardant in high-density polyethylene and added water and a glycol compound to prepare the thermal fluid used. The glycol compound is used here as antifreeze . 3M Innovative Properties Co. was recently awarded a patent for the elements and the process of an encapsulated fire retardant.
Also, the invention is mostly discussed in light of EV batteries. A battery when called upon to power an electric vehicle heats up. Thermal fluid flows through the container and over the modules of the battery. Porche AG, Volkswagen, and Audi AG even filed a patent on the same.
In the event of an overcharge, or a car accident resulting in a battery puncture, the flame retardant in the thermal fluid acts to reduce the fire hazard. More precisely, because of excess heat of the fire, bromine compound microcapsules rupture on reaching the rupture temperature. The flame retardant is released from the microcapsules and acts to bring the fire under control.
Thermal Runaway Of A Li
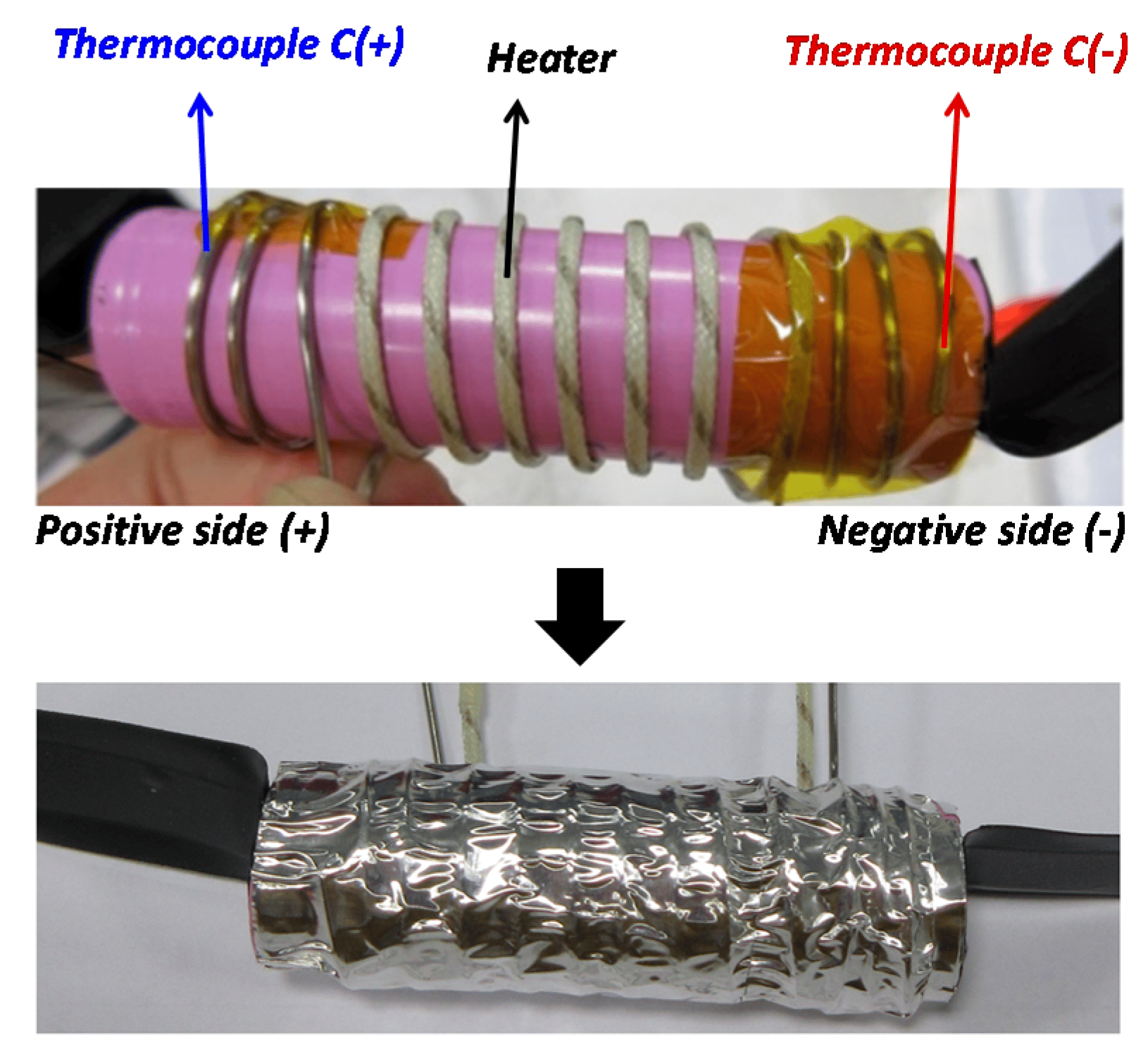
Drasti Patel1, James B. Robinson1,2, Sarah Ball3, Daniel J. L. Brett1,2 and Paul R. Shearing4,1,2
1Electrochemical Innovation Laboratory, Department of Chemical Engineering, University College London, WC1E 7JE, United Kingdom
2The Faraday Institution, Harwell Science and Innovation Campus, Didcot, OX11 0RA, United Kingdom
3Johnson Matthey Battery Materials, Oxford Science Park, Oxford, OX4 4GB, United Kingdom
You May Like: How Long Do Ryobi Batteries Last
The Threat Of Thermal Runaway
If youve flown in the last couple decades, youve surely been made aware of the danger of lithium-ion batteries aboard aircraft: spontaneous combustion of laptop batteries in the baggage hold, smartphones sandwiched between the seats catching fire . But what about lithium-ion batteries provokes such misbehavior? It actually comes down to physics, chemistry, and one major inherent limitation present in many battery configurations thermal runaway. In fact, the threat of thermal runaway remains a large safety concern not only for airlines but for a myriad of other industries, including the burgeoning battery electric vehicle sector. But while the unpredictability of a lithium-ion battery catastrophe can instill fear in the average consumer, the chemistry and physics of thermal runaway are not a mystery. In fact, its a fairly well understood process. Lets dive in.
A car battery that underwent thermal runaway. : Adobe Stock
Stage 2 Ramp Or Acceleration
Decomposition of the Solid-Electrolyte Interface starts exposing the reactive portion of the anode to exothermic reactions with the electrolyte. As the electrolytes are reduced at the anode due to the deteriorating SEI, they simultaneously oxidized as well. This is the stage where self-heating becomes prominent and rises almost linearly with the increasing temperature. If this heat is not dissipated by the battery structure, the elevated temperature will cause increasingly severer exothermic reactions. The rate of the self-heating increases linearly as a function of the temperature itself. On a plot of the self-heating rate vs temperature, this region shows up as a Ramp – it is called the ramp or acceleration region. The reaction causing this accelerated heat generation depends on the state of charge and the chemistry of batterys materials. In this stage, venting and smoke may also be observed. Timely intervention may arrest the reactions, and it is possible to avoid Stage 3, Thermal Runaway. Once Thermal Runaway has started, it cant be stopped, and the occurrence of thermal runaway may take minutes or even hours or days depending on the battery design, materials, and the operating environment.
Also Check: Replace Audi Key Fob Battery
Example Battery Enclosure Venting Mechanisms
One example of a venting mechanism is the PRO-LP rupture disc. This reverse acting disc is designed to burst accurately at the low pressures associated with battery enclosures. It is scored around the periphery of the disc, so if pressure increases to a critical point, the disc will break at the score to offer immediate pressure relief. Additionally, the PRO-LPâs low-profile, high-integrity design meets specifications on protrusion and offers immediate and full opening for rapid venting to reduce the risk of battery runaway propagation.
An alternative solution with an even lower profile is the Flat Composite Disc. Protrusion is minimal, and the forward acting composite disc will burst accurately at ultra-low pressures without compromising design integrity.
Finally, for larger battery applications , where additional venting area is needed, our MV range of explosion vents offer low-profile venting with the flexibility of being available in custom sizes, shapes and pressures to fit your requirements. Many of these vents require no frame, therefore reducing the overall cost while mitigating the risks associated with overpressurisation. The vent design further supports enhanced reliability and performance.
To learn more about the options for protecting your lithium-ion cells and battery packs, contact our specialist team on / 001 258-5626.
Indigenous Land Acknowledgement Statement
Lithium batteries are in products we use every day and can be dangerous on aircraft if not packed or shipped properly. This video was created for passengers, air carrier employees, gate agents, shippers, and consumers to reduce the risk of fire on an aircraft, especially in the cabin and flight deck by educating about the risk posed by lithium. Oct 25, 2018 · What you describe will be classified as a hazardous material when offered for transportation as: UN3481, Lithiumionbatteries contained in equipment, 9 A lithiumionbattery of 144 Wh while not below the initial threshold of 100 Wh is subject to the smaller lithiumbattery exception per 49 CFR 173.185 which has a threshold of 300 Wh.. The Watt-hour rating may not exceed 20 Wh for a lithium ion cell or 100 Wh for a lithium ion battery. After December 31, 2015, each lithium ion battery subject to this provision must be marked with the Watt-hour rating on the outside case..
sams club giftcard
Read Also: Toyota Camry Hybrid Battery Location
The Dangers Of Thermal Runaway
Thermal runaway may occur if a battery suffers abuse, resulting in the release of toxic and flammable gases. Thermal runaway occurring in a single battery cell can quickly spread, causing a cascading of thermal runaway in adjacent battery cells. Thermal runaway could culminate in a catastrophic high heat release fire event.
Lithium-ion battery fires are notoriously challenging to fight. Gaseous suppression and water systems simply are not effective. While fire suppression systems can slow fire growth and heat release, they are not sufficient to provide complete extinguishment once thermal runaway has started. The most effective method of extinguishing these types of fires requires large amounts of water applied for many hours or even days. In many locations, especially those that are remote or where water is scarce, this is not desirable or even achievable.
Unfortunately, there have been a number of these fire events in the last few years. In November of 2017, a fire at a Belgium grid-connected lithium-ion battery energy storage site near Brussels resulted in a cloud of toxic fumes that forced thousands of residents to stay at home. In April of 2019, a lithium-ion battery system exploded at an Arizona Public Service site, severely injuring eight firefighters. Following the catastrophe, U.S. energy utilities made safety a key focus. And between 2017 and 2019, there were 28 ESS fires in Korea, resulting in the suspension of 522 ESS facilities.